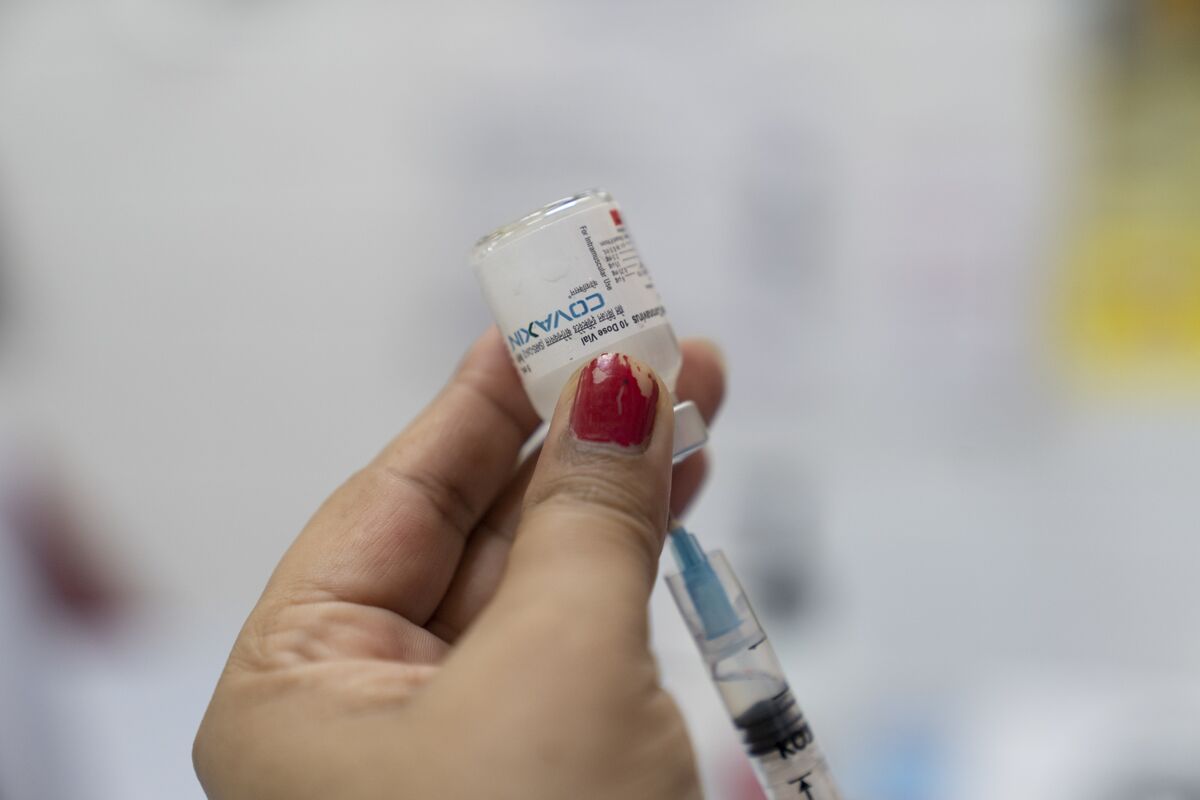
The World Health Organization granted emergency authorization to a Covid-19 vaccine co-developed by India’s medical-research agency and local manufacturer Bharat Biotech International Ltd., ending a months-long wait that added to controversy around the homegrown shot.
The WHO approved the vaccine’s use in people aged 18 and older on a two-dose schedule with four weeks between shots, according to a statement on Wednesday. Covaxin joins a range of WHO emergency-cleared shots from AstraZeneca Plc, China’s Sinopharm Group Co. and Sinovac Biotech Ltd., Pfizer Inc. and BioNTech SE , Johnson & Johnson and Moderna Inc.
"emergency" - Google News
November 03, 2021 at 07:34PM
https://ift.tt/3EEXR4p
WHO Approves India's Homegrown Covid Vaccine for Emergency Use - Bloomberg
"emergency" - Google News
https://ift.tt/2VVGGYQ
https://ift.tt/3d7MC6X
emergency
Bagikan Berita Ini














0 Response to "WHO Approves India's Homegrown Covid Vaccine for Emergency Use - Bloomberg"
Post a Comment